| List of possible results | |
| E4/E4 | Individuals with E4/E4 are far more likely to develop Alzheimer's and develop it earlier than the general population |
| E2/E4, E3/E4 | E2/E4 and E3/E4 are at higher risk of developing Alzheimer's than the general population |
| E2/E3, E3/E3 | E2/E3 and E3/E3 individuals are at a lower risk of developing Alzheimer's than the general population |
| E2/E2 | Alzheimer's is not common amongst those with E2/E2 and develop it later than the general population |
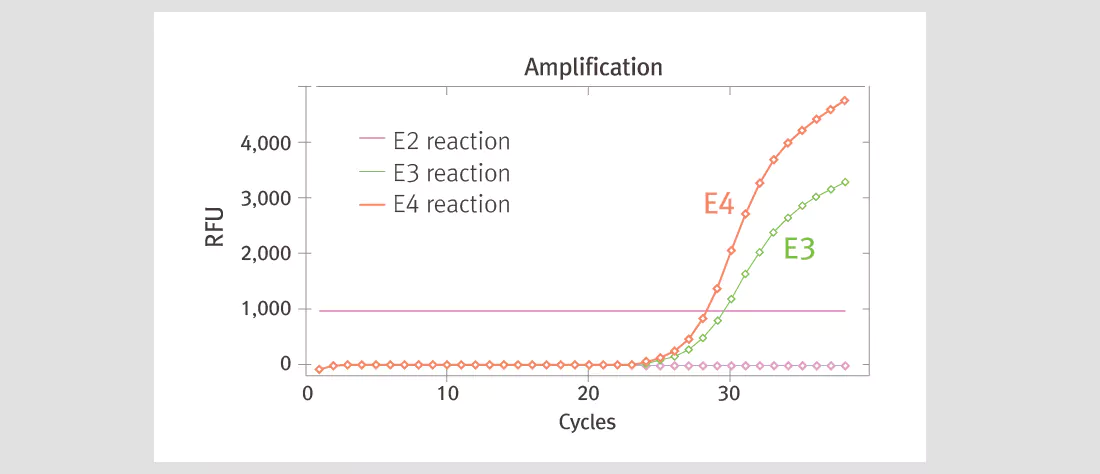
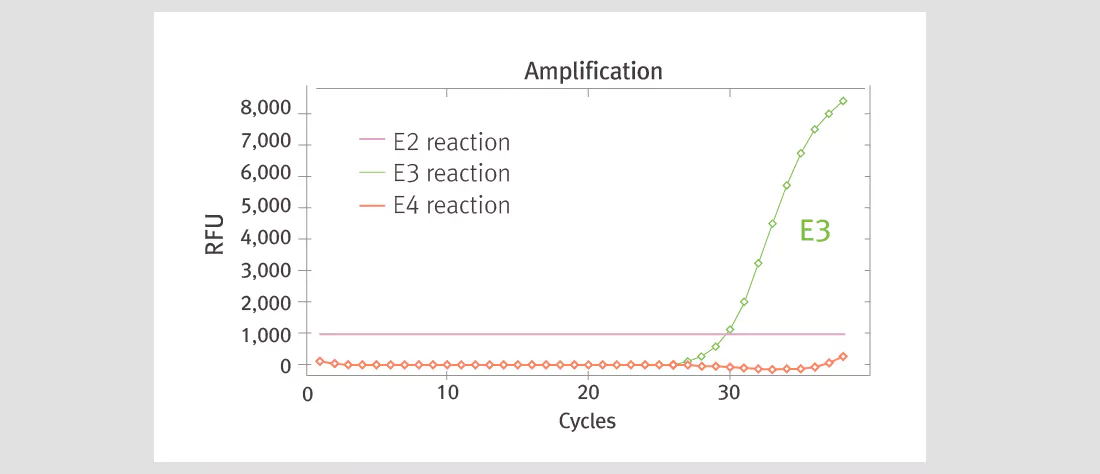
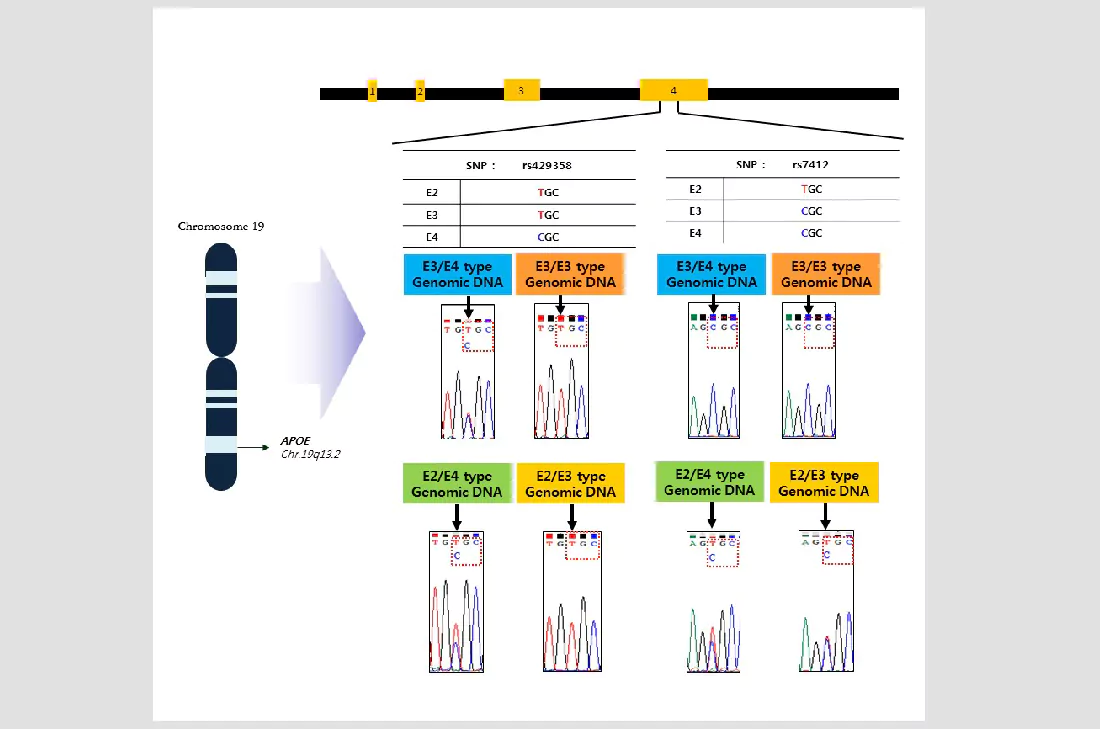

| Product Details | ||||
| Product Name | SKU | Test Quantity per Kit | Certifications | Qualifications |
| MF ApoE Kit | MF2020 | 96 individual tests | CE Mark | K-GMP, ISO 13485 |